The use of oil in industry. The use of oil. Modern classification systems can be built on three principles: hierarchical, faceted and mixed.
Petroleum products and their scope
PETROLEUM PRODUCTS- mixtures of various gaseous, liquid and solid hydrocarbons obtained from oil and oil associated gases. They are divided into the following main groups:
Fuel
Petroleum oils
Petroleum solvents
Solid hydrocarbons
Oil bitumen
Other petroleum products
Fuels include hydrocarbon gases, gasolines, jet fuels, diesel fuels, boiler fuels, etc.
Petroleum oils are heavy distillate and residual oil fractions subjected to special purification. They are divided into lubricating oils and oils for special purposes. The latter are used for technological purposes and in the operation of mechanisms. These include: electrical insulation - transformer, capacitor, cable; for hydraulic systems; for technological purposes - quenching and liquid absorbers, softeners, etc.; for pharmacopoeia and perfumery (white oils).
As solvents, narrow gasoline and kerosene fractions obtained by direct distillation of oil are used. Solvents are used in the rubber industry, for the preparation of glue, the extraction of oils from seeds and cakes, the manufacture of varnishes and paints, the production of polyvinyl chloride, etc. Lighting kerosene - straight-run kerosene fractions used in lighting and incandescent lamps and as household fuel.
Solid hydrocarbons include paraffin, ceresin and ozocerite and their mixtures with oils.
Bitumens are solid or viscous liquid substances obtained from the residual products of oil refining (from residues after the distillation of resinous oils, from tars, etc.).
Other oil products include: petroleum coke, greases, technical carbon, hydrocarbons (benzene, toluene, xylenes, etc.), as well as acidol (including soap naphtha), various fractions of oil distillation and products of their processing (in particular, alkylate , petroleum resins), etc.
A distinction is usually made between light and dark oils. The former include aviation and motor gasolines, solvent gasolines, jet fuel, kerosenes, diesel fuels, the latter include fuel oil, as well as distillate oils and tar obtained as a result of its distillation.
Part of commercial petroleum products is produced directly from oil or various oil fractions and residues; many petroleum products (eg, auto and aviation gasolines, boiler fuels, oils) are obtained by mixing (compounding) individual components-products of oil refining. Mixing of components allows to produce a commercial product of the required quality and at the same time rationally use the properties of each component.
End products of oil refining
Refineries blend petroleum products, add the necessary additives, provide short-term storage, and prepare them for loading onto trucks, barges, ships, and wagons.
gaseous fuel, such as propane, is loaded and transported to consumers in pressurized liquid form in specialized wagons.
Liquid fuel undergoes mixing (automotive and aviation fuel, kerosene, various types of fuel for aviation turbines, diesel fuels are obtained by adding color additives, detergents, anti-knock additives, oxygenates and fungicidal additives in appropriate proportions). Delivered by tankers, barges, trains and tankers. It can be transported to the consumer through pipes, for example jet fuel to an airport or to a supplier in multi-product pipes.
Lubricants(light machine oils, engine oils and various lubricants are obtained by adding viscosity stabilizers to required quantities) are usually transported in bulk to an adjacent packing station.
Paraffin, is used, among other things, in the packaging of frozen foods. It can be delivered in bulk form for further packing into blocks.
Sulfur(or sulfuric acid), by-products of oil refining, may be present up to a few percent as organic sulfur-containing inclusions. Sulfur and sulfuric acid- useful industrial materials. Sulfuric acid is usually transported and supplied as the acidic precursor oleum.
loose tar is delivered to packaging plants for further use in multi-layer soft roofing with an upper covering layer of tar concrete and other needs.
Asphalt- used as a binder for crushed stone in the manufacture of asphalt concrete, which is used in the construction of roads, etc. It is transported and supplied in bulk.
Petroleum coke, is used in various carbon products such as some types of electrodes and solid fuels.
petrochemicals or petrochemical raw materials are often sent for further processing. The petrochemicals may be olefins, their precursors, or various types of aromatic petrochemicals.
Petrochemicals have a wide range of applications. They are often used as monomers or raw materials for their manufacture. Olefins such as alpha-olefins and dienes are often used as monomers, aromatic hydrocarbons can also be used as monomer precursors. The monomers then undergo polymerization into different kinds of polymers.
The polymeric materials may be plastics, elastomers or fibers. Some polymers are used as gels and lubricants. Petrochemicals also find applications as solvents or raw materials for their production, precursors for a wide range of substances such as machine fluids, surfactants for cleaners, etc.
Physical and chemical properties of petroleum products
To assess the quality of petroleum products, a number of their physicochemical properties are determined.
Among the most important physical properties include: viscosity, density and fractional composition. To establish the latter, petroleum products are distilled at a strictly defined speed from a flask of standard shapes and sizes. The fractional composition is presented as a relationship between the temperature of the vapors of petroleum products in the flask and the amount of condensate (oil products condensed in the refrigerator and collected in the receiver). For gasolines, five points are usually given: the initial boiling point and the boiling points of 10%, 50%, 90% and 97.5% of the fuel. For some other petroleum products, eg. diesel fuels, often indicate the amount of substance that boils up to a certain specified temperature, e.g. up to 360 °C The fractional composition of oils is usually determined under reduced pressure (in vacuum) in order to avoid the decomposition of high-boiling fractions at their boiling points.
They also measure the pressure (elasticity) of vapors (ch. arr. for gasoline) in a steel bomb at a ratio of the volumes of the liquid and vapor phases 1:4 at 38 ° C. Usually in technical conditions limit the top. the value of vapor pressure, as a measure to prevent the formation of "vapor locks" in the fuel system of the engine.
The cloud point is determined (for motor fuels), at which crystals of high-melting hydrocarbons or water begin to separate from the fuel; the pour point (for oils, residual boiler fuels, diesel and jet fuels and aviation gasolines), at which the oil thickens under experimental conditions so much that its level in the test tube remains stationary for 1 min when tilted at an angle of 45o; flash point; ignition temperature; the melting point of solid petroleum products (paraffin, ozocerite, etc.), which corresponds to the moment of complete solidification (crystallization) of the pre-melted product.
Color characterizes the quality of purification of petroleum products from resinous and other colored substances; while the color of petroleum products is compared with the color of specially colored glasses.
Ductility, or extensibility, of bitumens characterizes their ability to stretch, without breaking, into thin threads under the influence of an applied force; defined in the spec. device (ductylometer) by stretching a bitumen sample of a standard form at a certain speed at 25 ° C.
To the most important chem. the properties of petroleum products include: the content of sulfur, tar, paraffin, and other indicators.
The sulfur content is determined in several ways. For light oil products, the lamp method is most common: a sample of the oil product is burned in a light bulb of known weight; the combustion products are taken up with a titrated NaHCO3 solution, the excess of which is titrated with an HCl solution. The method is sometimes used for dark oil products, which are pre-diluted with some light oil product with a known sulfur content. More often, a sample of a dark oil product is burned in a calorimetric bomb in an O2 atmosphere, and the amount of SO42- ions formed is determined gravimetrically after their precipitation with Ba chloride. The presence of aggressive sulfur compounds in petroleum products, in particular elemental sulfur and mercaptans, is detected by the change in color of the copper plate after its contact with the tested petroleum product. Sometimes the so-called doctoral test is used when a change in the color of elemental sulfur is observed under the influence of the products of interaction with Na2PbO2 of mercaptans and H2S present in the oil product.
The content of resins is determined by separating them from petroleum products by adsorption on some solid adsorbent (most often on silica gel) followed by desorption with a suitable extractant, a mixture of ethanol and benzene. In some oils and heavy residual fuels, the so-called excise resins are determined - substances capable of reacting with H2SO4 concentrate under strictly regulated experimental conditions. In gasoline, jet and diesel fuels, the amount of actual resins is determined, for which a sample of the fuel is evaporated in a jet of air or water vapor, and the remainder is weighed.
The paraffin content is set as follows: a sample of the oil product is dissolved in a suitable solvent, in gasoline, the solution is cooled to a temperature of -20 to -40 ° C and solid hydrocarbons are precipitated with ethanol or propanol. The precipitate is separated on a filter cooled to a given temperature, washed with a mixture of ethanol and gasoline to remove oil, and dissolved in petroleum ether. The latter is distilled off and the residue is weighed.
Oxidation resistance of gasolines and some other products is characterized by the value of induct. period-interval of time during which the tested oil product, which is in an O2 atmosphere under a pressure of 0.7 MPa at 100 ° C, practically does not oxidize. The oxidation resistance of some jet fuels is evaluated by the amount of sediment formed during its liquid-phase oxidation in a special device for 4 hours at 150 ° C, motor oils - by the change in the mechanical properties of a thin film of oil that is on a metal surface in contact with air at 260 ° C .
The corrosive activity of oils is evaluated by the change in the mass (g/m2) of a metal plate when it is exposed to a test oil heated to 140°C for 50 h, the layer of which is periodically in contact with atmospheric oxygen. The corrosive properties of fuels are usually judged by the presence or absence of active sulfur compounds in them, which is determined using a copper plate.
Carbonization- the ability of an oil product to form a carbonaceous residue (coke) during the evaporation of an oil product in a standard device and under strictly defined heating conditions; determined mainly for engine and cylinder oils, heavy residual fuels, 10% residue from the distillation of diesel fuels, as well as for raw materials of catalytic processes. and thermal. cracking, production of petroleum coke and bitumen, etc.
The height of a non-smoky flame characterizes the lighting and heating ability of light oil products (illuminating kerosenes, jet and diesel fuels) when burned in lamps, heated. appliances, etc. This indicator depends on the group chemical composition of petroleum products and, above all, on the content of aromatic hydrocarbons. The test sample is burned in a special lamp. design and measure the maximum smokeless flame height.
There are also a number of indicators that determine the consumer properties of petroleum products. These include, in particular, indicators of the knock resistance of gasolines (octane number) and the flammability of diesel fuels (cetane number).
Work description
PETROLEUM PRODUCTS - mixtures of various gaseous, liquid and solid hydrocarbons obtained from oil and oil associated gases. They are divided into the following main groups:
-Fuel
- petroleum oils
- petroleum solvents
-solid hydrocarbons
Oil production and refining has been an important part of the global economy for two centuries now. This is an important topic that many people are interested in. But not everyone knows what areas and applications of oil exist. We will talk about this in this article.
Oil is black gold
Oil, like many other substances, became known to man many centuries ago. But it was oil that was nicknamed "black gold" for its invaluable properties and ability to be processed. As a result of oil refining, a lot of useful products and materials that have found their application in various fields. What is this substance?
Oil is a combustible liquid with an oily structure. In nature, oil of various colors can be found. Yes, in most cases it is a dark brown or black liquid, but yellow, green or generally white (the so-called "white oil") is also found. This substance consists of liquid hydrocarbons, nitrogen elements, organic acids and many other chemical compounds.
Spheres and fields of application of oil
Almost every product or product that we use contains petroleum products in its composition. And in order to prove this, we will describe the most popular areas and applications of oil.
- Different types of fuel are the main product of oil refining. Oil refining includes many complex interrelated processes, as a result of which liquid fuel(gasoline, diesel fuel, kerosene and fuel oil) and raw materials for further processing.
- The second place is rightfully occupied by plastic (plastic). Every day we use plastic containers, various bags and plastic bags. They are all made from oil. Plastic is very convenient, as it easily takes any shape and has properties useful for the production of household items.
- Synthetic fabrics. On the this moment, there are different artificial fibers (nylon, acrylic, polyester) from which clothes are made. They have excellent properties for the production of a wide variety of clothing, from underwear to shoes.
- Oil is also used in its raw, unrefined form to build pipelines and power lines.
- Synthetic rubbers. Oil refining products serve as raw materials for the production of this substance. Rubber is subsequently used to make tires for various vehicles.
- Solar panels. Panels on which photovoltaic cells are applied to convert solar energy are produced from petroleum products.
- Food products. They learned to produce synthetic protein from oil, which became a cheaper substitute for animal protein. Paraffin resins, which are also derived from petroleum, are used to make chewing gum.
The use of oil in medicine and cosmetology
Medicine also cannot do without oil. Many medicines are made from hydrocarbons - derivatives of petroleum products. These drugs include aspirin, phenyl salicylate, petroleum jelly, streptocide, antibiotics, various antiseptics and antiallergic substances.
Cosmetic products (nail polishes, eye and lip pencils, lipstick) contain propylene glycol, minerals, parabens. These substances are products of oil refining. For example, lipsticks contain ceresin, liquid and solid paraffins, which are also petroleum products. They are introduced to moisturize the skin and increase the shelf life, to give viscosity and the desired consistency to cosmetic products.
Oil is a resource in demand all over the world. As you can see, it is used in a variety of areas, from the fuel industry to the food industry. Life without oil is no longer imaginable, and with the development of science and technology, the scope and scope of oil will probably expand.
Rapid scientific and technological progress and high rates of development of various branches of science and the world economy in the XIX - XX centuries. led to a sharp increase in the consumption of various minerals, a special place among which was occupied by oil. Oil began to be extracted on the banks of the Euphrates in 6-4 thousand years BC. It was also used as a medicine. The ancient Egyptians used asphalt (oxidized oil) for embalming. Petroleum bitumen was used to prepare mortars. Oil was part of the "Greek fire". In the Middle Ages, oil was used for lighting in a number of cities in the Middle East, Southern Italy, etc. At the beginning of the 19th century. in Russia, and in the middle of the XIX century. In America, kerosene was obtained from oil by sublimation. It was used in lamps. Until the middle of the XIX century. oil was extracted in small quantities from deep wells near its natural outlets to the surface. The invention of the steam engine, and then the diesel and gasoline engine, led to the rapid development of the oil industry.
Oil is a liquid natural mixture of various hydrocarbons with small amounts of other organic compounds; a valuable mineral, often occurring together with gaseous hydrocarbons; oily, flammable liquid with a characteristic odor, usually Brown color with a greenish or other tinge, sometimes almost black, very rarely colorless.
Oil is a rock. It belongs to the group of sedimentary rocks along with sands, clays, limestones, rock salt, etc. We are used to thinking that rock is a solid substance that makes up the earth's crust and deeper bowels of the Earth. It turns out that there are liquid rocks, and even gaseous ones. One of the important properties of oil is the ability to burn.
Composition of oil
By composition, oil is a complex mixture of hydrocarbons of various molecular weights, mainly liquid (solid and gaseous hydrocarbons are dissolved in them). Depending on the field, oil has a different qualitative and quantitative composition. Oil consists mainly of carbon - 79.5-87.5% and hydrogen - 11.0-14.5% by weight of oil. In addition to them, oil contains three more elements - sulfur, oxygen and nitrogen. Their total amount is usually 0.5-8%. In insignificant concentrations in oil there are elements: vanadium, nickel, iron, aluminum, copper, magnesium, barium, strontium, manganese, chromium, cobalt, molybdenum, boron, arsenic, potassium. Their total content does not exceed 0.02-0.03% by weight of oil. These elements form organic and inorganic compounds that make up oil. Oxygen and nitrogen are found in oil only in a bound state. Sulfur can occur in the free state or be part of hydrogen sulfide.
The composition of oil includes about 425 hydrocarbon compounds. The main part of the oil is made up of three groups of hydrocarbons: methane, naphthenic and aromatic. Along with hydrocarbons, oil contains chemical compounds of other classes. Usually all these classes are combined into one group of heterocompounds (Greek "heteros" - another). More than 380 complex heterocompounds have also been found in oil, in which elements such as sulfur, nitrogen and oxygen are attached to hydrocarbon cores. Non-hydrocarbon compounds are also isolated in oil: the asphalt-resinous part, porphyrins, sulfur and the ash part. Oxygen in oil is also found in a bound state in the composition of naphthenic acids (about 6%) - CnH2n-1 (COOH), phenols (not more than 1%) - C6H5OH, as well as fatty acids and their derivatives - C6H5O6 (P). The nitrogen content in oil does not exceed 1%, the resin content can reach 60% by weight of oil.
Oil formation
In recent years, thanks mainly to the work of geologists, chemists, biologists, physicists and researchers of other specialties, it has been possible to elucidate the main regularities in the processes of oil formation. It has now been established that oil of organic origin, i.e. it, like coal, arose as a result of the transformation of organic substances. The process of oil formation began many millions of years ago with the development of life and continues to this day. Oil is classified as a non-renewable energy source, a person is not able to create a new oil field in a short time.
Oil and combustible gas accumulate in porous rocks called reservoirs. A good reservoir is a sandstone bed embedded in impermeable rocks such as clays or shales that prevent oil and gas from leaking from natural reservoirs. The most favorable conditions for the formation of oil and gas deposits occur when the sandstone layer is bent into a fold, facing upwards. In this case, the upper part of such a dome is filled with gas, oil is located below, and even lower - water.
Scientists argue a lot about how oil and combustible gas deposits were formed. Some geologists - supporters of the hypothesis of inorganic origin - argue that oil and gas deposits were formed as a result of seepage from the depths of the Earth of carbon and hydrogen, their combination in the form of hydrocarbons and accumulation in reservoir rocks. Other geologists, most of them, believe that oil, like coal, arose from organic matter buried deep under marine sediments, where combustible liquid and gas were released from it. This is an organic hypothesis of the origin of oil and combustible gas. Both of these hypotheses explain part of the facts, but leave the other part unanswered.
There were different opinions on the source material. Some scientists believed that oil arose from the fats of dead animals (fish, plankton, etc.), others believed that leading role squirrels played, third gave great importance carbohydrates. It has now been proven that oil can be formed from fats, proteins and carbohydrates, i.e. from all organic matter. Oil is formed under the surface of the earth during the decomposition of marine organisms. The remains of tiny microorganisms that lived in the sea and, to a lesser extent, those that lived on land and were carried to the sea by the waves of rivers, plants growing on the ocean floor - all this is mixed with sand and silt that rest on the ocean floor. Such places, rich in organic constituents, become the source rock for the formation of crude oil.
Gradually, the sediments become thicker and thicker and, under their own weight, sink deeper and deeper into the seabed. As new layers accumulate from above, the pressure on the lower layers increases several thousand times, and the temperature rises several hundred degrees, mud and sand harden into shale and sandstone, carbonate sediment and shell remains form limestone, and the remains of dead organisms transform into crude oil and natural gas.
As soon as oil forms, it begins to move upward, closer to the earth's surface, because the density of oil is less than the density of sea water, which fills the cracks in the rocks, sands and rocks that form the earth's crust. Natural gas and crude oil seep into the microscopic pores of the formations above. Sometimes it happens that oil gets into impermeable layers of sediments or surrounded by a thick layer of rock that does not allow it to move on. Oil gets trapped, and thus oil fields are formed.
Oil production
Oil has been extracted by mankind since ancient times. At first, primitive methods were used: collecting oil from the surface of reservoirs, processing sandstone or limestone soaked with oil using wells. The first method was used in Media and Syria, the second - in the 15th century in Italy. But the beginning of the development of the oil industry is considered to be the time when mechanical drilling of wells for oil appeared in 1859 in the United States, and now almost all the oil produced in the world is extracted through boreholes. Over a hundred years of development, some fields have been depleted, others have been discovered, the efficiency of oil production has increased, oil recovery has increased, i.e. completeness of oil recovery from the reservoir. But the structure of fuel production has changed.
The main machine for oil and gas production is a drilling rig. The first drilling rigs, which appeared hundreds of years ago, essentially copied the worker with a crowbar. Only the scrap of these first machines was heavier and more like a chisel in shape. That's what it was called - a drill bit. He was hung on a rope, which was then raised with the help of a gate, then lowered. Such machines are called shock-rope. They can be found in some places even now, but this is already yesterday's day of technology: they punch a hole in the stone very slowly, they waste a lot of energy in vain.
Much faster and more profitable is another method of drilling - rotary, in which the well is drilled. A thick steel pipe is suspended from an openwork metal four-legged tower as high as a ten-story building. It is rotated by a special device - a rotor. At the lower end of the pipe is a drill. As the well gets deeper, the pipe is lengthened. So that the destroyed rock does not clog the well, a clay solution is pumped into it through a pipe with a pump. The solution flushes the well, carries away destroyed clay, sandstone, limestone up the gap between the pipe and the walls of the well. At the same time, the dense fluid supports the walls of the well, preventing them from collapsing.
But rotary drilling also has its drawbacks. The deeper the well, the harder it is for the rotor motor to work, the slower the drilling progresses. After all, it is one thing to rotate a pipe 5-10 m long when drilling is just beginning, and it is quite another to rotate a pipe string 500 m long. But what if the well depth reaches 1 km? 2 km? In 1922, Soviet engineers M. A. Kapelyushnikov, S. M. Volokh and N. A. Kornev built the world's first machine for drilling wells, in which it was not necessary to rotate the drill pipes. The inventors placed the engine not at the top, but at the bottom, in the well itself - next to the drilling tool. Now all the power of the engine was spent only on the rotation of the drill itself. This machine and the engine was extraordinary. Soviet engineers forced the same water, which previously only washed out the destroyed rock from the well, to rotate the drill. Now, before reaching the bottom of the well, the mud turned a small turbine attached to the drilling tool itself.
The new machine was called a turbodrill, over time it was improved, and now several turbines mounted on one shaft are lowered into the well. It is clear that the power of such a "multiturbine" machine is many times greater and drilling is many times faster. Another remarkable drilling machine is an electric drill invented by engineers A.P. Ostrovsky and N.V. Aleksandrov. The first oil wells were drilled with an electric drill in 1940. With this machine, the pipe string also does not rotate, only the drilling tool itself works. But it is not a water turbine that rotates it, but an electric motor placed in a steel jacket - a casing filled with oil. The oil is under high pressure all the time, so the surrounding water cannot enter the engine. In order for a powerful engine to fit in a narrow oil well, it was necessary to make it very high, and the engine turned out to be like a pillar: its diameter is like that of a saucer, and its height is 6-7 m.
Drilling is the main work in oil and gas production. Unlike, say, coal or iron ore oil and gas does not need to be separated from the surrounding massif by machines or explosives, it does not need to be raised to the surface of the earth by a conveyor or in trolleys. As soon as the well has reached the oil-bearing formation, the oil, compressed in the depths by the pressure of gases and groundwater, itself rushes up with force. As the oil pours out to the surface, the pressure decreases and the remaining oil in the subsoil stops flowing upward. Then, through wells specially drilled around the oil field, water is injected. Water puts pressure on the oil and squeezes it to the surface along the newly revived well. And then there comes a time when only water can no longer help. Then a pump is lowered into the oil well and oil is pumped out of it.
Oil refining
Alkylation appeared in 1930. In the process of alkylation, small molecules obtained by thermal cracking are rearranged under the action of a catalyst. As a result, branched-chain molecules are formed in the boiling zone of gasoline, which have higher performance, for example, increased anti-knock ability, fuel that ensures the operation of modern aircraft engines has such an ability.
Cracking. Cracking is the process of splitting hydrocarbons contained in oil, as a result of which hydrocarbons with a smaller number of carbon atoms in the molecule are formed. The yield of gasoline from oil can be significantly increased (up to 65-70%) by splitting long-chain hydrocarbons contained, for example, in fuel oil, into hydrocarbons with a lower relative molecular weight. This process is called cracking (from the English. Crack - split). Cracking was invented by the Russian engineer V. G. Shukhov in 1891. In 1913, Shukhov's invention began to be used in America. Currently, in the US, 65% of all gasoline is produced at cracking plants. At cracking plants, hydrocarbons are not distilled, but split. The process is carried out at higher temperatures (up to 600o), often at elevated pressure. At such temperatures, large hydrocarbon molecules are broken up into smaller ones.
Fuel oil is thick and heavy, its specific gravity is close to unity. This is because it is made up of complex and large hydrocarbon molecules. When fuel oil is cracked, some of its constituent hydrocarbons are broken down into smaller ones. And light oil products - gasoline, kerosene - are made up of small hydrocarbons. Fuel oil is the residue of the primary distillation. At the cracking plant, it is again processed, and from it, as well as from oil at the primary distillation plant, gasoline, naphtha, kerosene are obtained. During primary distillation, oil undergoes only physical changes. Light fractions are distilled off from it, i.e., parts of it are selected, boiling at low temperatures and consisting of hydrocarbons of different sizes. The hydrocarbons themselves remain unchanged.
During cracking, oil undergoes chemical changes. The structure of hydrocarbons is changing. In the apparatus of cracking plants, complex chemical reactions take place. These reactions are enhanced when catalysts are introduced into the apparatus. One such catalyst is specially treated clay. This clay in a finely crushed state - in the form of dust - is introduced into the plant's equipment. Hydrocarbons, which are in a vaporous and gaseous state, are combined with clay dust particles and crushed on their surface. Such cracking is called pulverized catalyzed cracking. This type of cracking is now widespread. The catalyst is then separated from the hydrocarbons. Hydrocarbons go their way to rectification and refrigerators, and the catalyst goes to its reservoirs, where its properties are restored. Catalysts are the biggest achievement of oil refining. Cracking plants of all systems produce gasoline, naphtha, kerosene, diesel oil and fuel oil. The focus is on petrol. They try to get more and necessarily better quality. Catalytic cracking appeared precisely as a result of the long-term, stubborn struggle of oilmen to improve the quality of gasoline.
Reforming- (from the English. Reforming - to remake, improve) an industrial process for processing gasoline and naphtha fractions of oil in order to obtain high-quality gasolines and aromatic hydrocarbons. In this case, the hydrocarbon molecules are basically not split, but converted. The raw material is the naphtha fraction of oil. Since the 40s, reforming has been a catalytic process, the scientific foundations of which were developed by N. D. Zelinsky, as well as V. I. Karzhev, B. L. Moldavsky. This process was first carried out in 1940 in the USA. It is carried out in an industrial plant with a heating furnace and at least 3-4 reactors at t 350-520 0 C, in the presence of various catalysts: platinum and polymetallic, containing platinum, rhenium, iridium, germanium, etc., in order to avoid deactivation of the catalyst by the compaction product coke, reforming is carried out under high pressure hydrogen, which circulates through the heating furnace and reactors. As a result of reforming gasoline fractions of oil, 80-85% gasoline with an octane number of 90-95, 1-2% hydrogen and the rest of the gaseous hydrocarbons are obtained. From a tubular furnace under pressure, oil is fed into the reaction chamber, where the catalyst is located, from here it goes to a distillation column, where it is separated into products. Reforming is of great importance for the production of aromatic hydrocarbons (benzene, toluene, xylene, etc.). Previously, the main source of these hydrocarbons was the coke industry.
Oil use
A variety of products of great practical importance are isolated from oil. In the beginning, dissolved hydrocarbons (mainly methane) are separated from it. After distillation of volatile hydrocarbons, the oil is heated. Hydrocarbons with a small number of carbon atoms in the molecule, having relatively low temperature boiling. As the temperature of the mixture rises, hydrocarbons with a higher boiling point are distilled. Thus, individual mixtures (fractions) of oil can be collected. Most often, with such a distillation, three main fractions are obtained, which are then subjected to further separation.
Currently, thousands of products are obtained from oil. The main groups are liquid fuels, gaseous fuels, solid fuels (petroleum coke), lubricating and special oils, paraffins and ceresins, bitumens, aromatic compounds, soot, acetylene, ethylene, petroleum acids and their salts, higher alcohols. These products include combustible gases, gasoline, solvents, kerosene, gas oil, domestic fuels, a wide range of lubricating oils, fuel oil, road bitumen and asphalt; this also includes paraffin, petroleum jelly, medical and various insecticidal oils.
Oils from petroleum are used as ointments and creams, as well as in the production of explosives, medicines, cleaning products, the greatest use of petroleum products is found in the fuel and energy industry. For example, fuel oil has almost one and a half times higher calorific value compared to the best coals. It takes up little space when burned and does not produce solid residues when burned. The replacement of solid fuels with fuel oil at thermal power stations, factories, and in railway and water transport yields enormous savings in funds and promotes the rapid development of the main branches of industry and transport.
The energy direction in the use of oil is still the main one in the world. The share of oil in the global energy balance is more than 46%. However, in recent years, petroleum products have been increasingly used as raw materials for the chemical industry. About 8% of the produced oil is consumed as a raw material for modern chemistry. For example, ethyl alcohol is used in about 150 industries. The chemical industry uses formaldehyde (HCHO), plastics, synthetic fibers, synthetic rubber, ammonia, ethyl alcohol, etc. Oil refinery products are also used in agriculture. Growth stimulants, seed disinfectants, pesticides, nitrogen fertilizers, urea, films for greenhouses, etc. are used here. In mechanical engineering and metallurgy, universal adhesives, plastic parts and parts of apparatus, lubricating oils, etc. are used.
Petroleum coke has found wide application as an anode mass in electric smelting. Pressed soot goes to fire-resistant linings in furnaces. In the food industry, polyethylene packaging, food acids, preservatives, paraffin are used, protein-vitamin concentrates are produced, the feedstock for which are methyl and ethyl alcohols and methane. In the pharmaceutical and perfume industries, ammonia, chloroform, formalin, aspirin, petroleum jelly, etc. are produced from petroleum derivatives. Petroleum synthesis derivatives are also widely used in the woodworking, textile, leather, footwear and construction industries.
Oil is the most valuable natural resource, which has opened amazing possibilities of "chemical reincarnation" for man. In total, there are already about 3 thousand oil derivatives. Oil occupies a leading place in the global fuel and energy economy. Its share in the total consumption of energy resources is constantly growing. Oil forms the basis of the fuel and energy balances of all economically developed countries. Currently, thousands of products are obtained from oil.
In the near future, oil will remain the basis for providing energy for the national economy and raw materials for the petrochemical industry. Here much will depend on success in prospecting, exploration and development of oil fields. But natural resources of oil are limited. The rapid increase in their production over the past decades has led to the relative depletion of the largest and most favorably located deposits.
In the problem of rational use of oil, an increase in the coefficient of their beneficial use. One of the main directions here involves deepening the level of oil refining in order to meet the country's demand for light oil products and petrochemical raw materials. Another effective direction is to reduce the specific fuel consumption for the production of heat and electricity, as well as the overall reduction in the specific consumption of electricity and heat in all sectors of the national economy.
Every time a person hears on the radio or reads in the newspaper that oil is getting more expensive, he gets the idea that fuel prices will rise again.
However, if you understand the process of recycling and refining oil, you will notice that the rise in oil prices affects not only fuel prices. Oil refining products are used to produce many things and objects with which a person has daily contact, and does not even suspect that the same kettle, toothbrush, TV was once just a “black liquid”.
Naturally, most of the extracted oil goes to the production of plastic (this is a polymer substance, which, in turn, is synthesized from monomers), which is part of, as mentioned above, a toothbrush, a plastic kettle, a TV case and monitors for computers, toys for children, insulation of cables, dishes, lamps, packaging, sports equipment. That is, for everything that consists of plastic or plastic.
It turns out that a person does not even think about the scale of oil use and how many “oil” items have passed through his hands today.
For example, oil is used to produce so-called ABS plastic, which, in turn, is used to make large car parts, to produce the sheath of many cables, toys, and shoe soles. Styrene, which is also a product of oil refining, is used to make office supplies, refrigerators, and even plumbing fixtures. 
The well-known polyethylene, which is now the most commonly used packaging material, was once oil. In addition, it is also used in the production of bottles and other containers.
Many people know that plexiglass is used to make glasses and many lighting fixtures. It is called polymethyl methacrylate, the precursor of which is also oil, which becomes polyurethane and is used in the manufacture of mattresses and shoe soles.
However, this was said only about plastic things, although oil also goes to the production of rubber things, such as tires for automobiles and other wheels, toys, gaskets for plumbing, sports equipment.
Also, various synthetic fabrics, which are formed by processing polymers, have tightly entered our lives. They are passed through special holes, while receiving synthetic strands, which are used to make the fabric. The most famous are polyamide fibers - for example, nylon, anide and enant - the range of use of which is quite wide: sewing threads, fishing nets, conveyor belts.
It should be noted that oil refining products are also used for the production of modern, fairly effective detergents and household chemicals. Their main advantage over natural remedies is their high efficiency and powerful action.
In addition, several interesting facts can be cited as an example. For example, at the University of Rhode Island, scientists have invented a plastic that can change its color at a certain temperature. At the moment it is 82 degrees. At this value, it turns yellow. 
It turns out that the possibility of using such plastic is practically unlimited. It can be used both for decorative purposes and for sanitary purposes (by color it will be possible to determine how long the products have been at a high temperature).
It should be noted that the world production produces about 200 million tons of plastic per year. Although the mass production of oil refining products (combs, buttons and toys) began only at the end of the 19th century.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Posted on http://www.allbest.ru/
FEDERAL AGENCY FOR EDUCATION
State educational institution of higher professional education
Siberian State Aerospace University named after Academician M.F. Reshetnev
COURSE WORK
By discipline: "Commodity science and expertise"
On the topic: Properties and application of petroleum products
Completed: st.gr TDZ-91/2 Malakhov L.V.
Krasnoyarsk 2010
Introduction
3. Basic information about oil
5. Certification and acceptance of goods
Conclusion
List of used literature
Introduction
The role of oil and natural gas in the world economy is exceptionally great. Oil, gas and products of their processing are used in almost all sectors of the national economy: transport and medicine, shipbuilding and agriculture, textile industry and energy. Oil and gas are mainly cheap sources of energy, but with the development of the chemical industry, they are increasingly being used as chemical raw materials. Now a wide variety of products are obtained from oil and gas: synthetic fibers, plastics, organic acids, gasolines, alcohols, synthetic solvents, and much more.
Oil is a natural mixture of hydrocarbons with an admixture of sulfur, nitrogen and oxygen compounds. It is a natural fossil fuel, but different from the rest great content hydrogen and the amount of heat released during combustion.
At present, three main directions for the use of oil have been identified: obtaining energy raw materials, obtaining materials with desired properties, and obtaining chemical and pharmaceutical products. Oil created not only new level productive forces of society, but also created a new science of petrochemistry, which arose at the junction of organic chemistry, petroleum chemistry and physical chemistry.
1. Current state oil products market
The main oil products - gasoline, diesel fuel and heating oil, are products of widespread and large-scale demand, the uninterrupted supply of which creates normal conditions for the life of the population and the development of society, the socio-economic and military-political stability of the state. In addition, gasoline, diesel fuel and heating oil occupy a very prominent place in the structure of Russian exports, ensuring the flow of foreign exchange reserves into the country.
The market for these petroleum products, being part of the markets for products of the fuel and energy complex, includes the domestic Russian wholesale market for industrial products, the market for consumer goods (according to experts, at present, the private sector consumes approximately 45-50% of all gasoline sold on the domestic market and enterprises , respectively, 50 - 55%) and the foreign trade (export-oriented) market. Therefore, the situation in all these segments of the commodity market affects the conjuncture of the oil products market.
Rice. one
In the first half of 2003, in the production and sale of petroleum products on the domestic Russian market, the trends that had taken shape last year were preserved. The state of the domestic oil products market was affected by the favorable situation for exporters on the world market, the general improvement in the state of the Russian economy, including an increase in the volume of transportation, as well as an increase in the material well-being of Russian citizens.
In January-May 2003, the Russian economy developed at high rates: industrial production amounted to 107.2% compared to the corresponding period of last year, transport freight turnover - 105.0%, real disposable cash income - 111.3%. This contributed to the growth of solvent demand for petroleum products. The development of the country's economy required a corresponding increase in production from the oil refining industry, while creating all the necessary prerequisites for this.
In January-May, the volume of oil production amounted to 111.3% against the level of the same period in 2002, and primary oil refining at Russian oil refineries amounted to 104.8%. Accordingly, the production of petroleum products increased: motor gasoline - 104.5%, diesel fuel - 103.5%, fuel oil - 105.1%.
Fig.2
The oil refining industry processed 46.6% of the oil and gas condensate produced in the Russian Federation, and the production of oil products using deepening technologies increased by 18.1%. Since the beginning of the year, the production of high-octane gasoline increased by 9%, and its share in the total production of motor gasoline increased from 46.9% to 48.8%.
According to the official data of the State Statistics Committee, 65 plants are involved in the production of petroleum products in the Russian Federation. A significant part of the production is exported. The average annual share of exports in the volume of production is: 10-15% for motor gasoline, 35-40% for diesel fuel, and more than 55% for fuel oil.
Among the regions, the largest producer of petroleum products is the Republic of Bashkortostan. Oil refineries of the Bashkir group produce from the volume of all-Russian production: motor gasoline - 20%, diesel fuel - 16%, fuel oil - 15%.
Russia's own production of motor gasoline, diesel fuel and heating oil not only fully meets the needs of the domestic market, but also supplies them significantly to the world market. The Russian oil refining industry has a huge potential - the total capacity of the plants is more than 260 million tons, but at present the volume of oil refining is less than 65% of the level of the late 80s and early 90s.
The reserve for increasing the yield of oil products is to increase the depth of oil refining. Currently, the average depth of refining at Russian refineries is about 70%, and in the United States, for example, about 90% (at the best American refineries it reaches 98%). It should be noted that such a situation with the depth of processing has developed historically, based on the need of the fuel balance of the USSR in fuel oil.
Currently, the oil refining industry has the resources necessary for development: skilled labor and stocks of raw materials (oil). The efficiency of the industry is hindered by the underfunding of the technical base. Further development of the complex requires modernization and technical re-equipment of production. The increase in refinery utilization is accompanied by both the construction of new production facilities and their reconstruction aimed at increasing the yield of high-quality petroleum products.
The consumption of petroleum products in the regions differs significantly both in absolute and relative terms. The provision of diesel fuel and motor gasoline both throughout the country and at the regional level remained fairly stable.
Prices for petroleum products this year remained stable and even declined. Thus, in May 2003, producer prices decreased in comparison with December 2002 - the price index amounted to 85.2%. In May, the consumer price index for gasoline amounted to 99.1% (from the beginning of the year 108.3%), the producer price index - 96.5% (from the beginning of the year 96.3%).
In May, a decrease in consumer prices for gasoline was noted in 46 constituent entities of the Russian Federation. The most significant it was in the Novosibirsk region. (7.7%). Compared to the previous month, gasoline prices remained unchanged in 33 constituent entities of the Russian Federation. The increase in gasoline prices was observed in nine constituent entities, with the largest increase in the republics of Sakha (Yakutia) and Dagestan (0.5%). In Moscow, consumer prices for gasoline remained virtually unchanged in May, while in St. Petersburg they fell by an average of 0.4%.
In May, on average in Russia, there was also a decrease in producer prices for motor gasoline of all brands by 3.5%, caused by its reduction in price in 14 regions of the Russian Federation (from 1.9% in Omsk, Nizhny Novgorod regions and the Komi Republic to 9.5% in the Republic of Bashkortostan and the Ryazan Region). In three constituent entities, an increase in prices for motor gasoline was noted (the maximum increase in prices was observed in the Orenburg region - by 2.6%). At the level of April, gasoline prices in five subjects of the Russian Federation remained.
In June 2003, motor gasoline prices were almost at the level of May.
AT last week June, the rise in the price of gasoline was noted in eight centers of the subjects of the Russian Federation. The largest increase in prices for motor gasoline was observed in Novosibirsk (by 16.4% on average), including for A-76 (AI-80, etc.) gasoline by 28.4%. In 72 centers of the constituent entities of the Russian Federation, prices for motor gasoline remained unchanged. Cheaper gasoline was noted in eight centers of the constituent entities of the Russian Federation. Among the territorial centers of Russia, the largest price reduction was observed in Belgorod (on average by 2.2%), where A-76 (AI-80, etc.) gasoline became cheaper by 2.6%. In Moscow and St. Petersburg, prices for motor gasoline remained at the level of the previous week.
Prospects for the development of the oil refining industry are set out in the Federal Target Program "Energy Efficient Economy for 2002-2005". and for the future up to 2010. (approved by Decree of the Government of the Russian Federation of November 17, 2001 No. 796). It provides for the implementation of a set of measures for the reconstruction of oil refining capacities in order to deepen oil refining, increase the production of high-quality petroleum products and reduce the cost of their production.
In the period up to 2005, it is envisaged to commission new installations that will increase the depth of oil refining, with a total capacity of about 30 million tons and 15 million tons of capacities that improve the quality of petroleum products, and in 2006-2010. about 10 and 9 million tons, respectively.
In addition, by 2005 it is planned to put into operation new facilities for the primary processing of 15.5 million tons of oil. As a result, the refining depth for the industry as a whole will be 73% in 2005 and 75% in 2010, with primary refining volumes of 200 and 210 million tons, respectively. their export.
The implementation of these measures will ensure a reduction in emissions of harmful substances into the environment, a reduction in energy and material costs in the production of products. Termination of the production of high-sulphur fuels will reduce emissions of sulfur oxides into the atmosphere by almost 2 times, and the introduction of modern large-tonnage oil refining processes will reduce irretrievable losses by 0.1-0.2% of the volume of refined oil. Due to the use of highly active and selective catalysts and economical modern equipment in the processing industry, the consumption of fuel, heat and electricity can be reduced in the industry as a whole by 5 million tons of reference fuel per year. As a result of the planned reconstruction of the oil refining industry, the total energy intensity of the refining industry will decrease from 10.4% in 2001 to 7.9% in 2005 and to 7% in 2010 in oil equivalent.
2. Properties and application of petroleum products by type
Oil refining is a complex multi-stage technological process, which results in a wide range of marketable products that differ in structure, physico-chemical properties, composition and applications; at oil refineries, after preliminary purification from mechanical impurities, desalting and dehydration, oil is sent for processing; one of the options:
1) according to the fuel option, oil enters atmospheric vacuum distillation, where, after repeated condensation and evaporation on the plates of the distillation column, the oil is separated into fractions, after rectification, light products are fractionally sent to hydrotreatment or catalytic reforming, and vacuum gas oil and tar to cracking; the yield of light oil products is 85% or more, depending on the composition of the processed oil;
2) according to the oil version, after the selection of light oil products, the residual fuel oil after rectification is sent to deep vacuum distillation at temperatures of 350-500 ° C, where oil distillates are isolated, which are subjected to complex purification and used to obtain commercial oils; according to m.v. also receive a number of valuable products for petroleum synthesis, construction and chemical industries.
Products produced at oil refineries are divided into the following groups, which differ in composition, properties and applications:
1) Asphalt
2) Diesel fuel
3) Fuel oil
4) Gasoline
5) Kerosene
6) Liquefied petroleum gas (LPG)
7) Petroleum oils
8) Paraffin
9) Lubricants
Asphalt (from the Greek. Yutsblft - mountain resin) - a mixture of bitumen (60-75% in natural and 13-60% in artificial) with mineral materials (crushed stone or gravel, sand and mineral powder). Used for coating highways as a roofing, hydro- and electrical insulating material, for the preparation of putties, adhesives, varnishes, etc. Asphalt can be of natural and artificial origin. Often the word asphalt refers to asphalt concrete - an artificial stone material, which is obtained as a result of the compaction of asphalt concrete mixtures. Classical asphalt concrete consists of crushed stone, sand, mineral powder (filler) and bituminous binder (bitumen, polymer-bitumen binder; tar was previously used, however, being extremely unecological, it is not currently used).
artificial asphalt
Artificial asphalt or asphalt mix is a building material in the form of a compacted mixture of crushed stone, sand, mineral powder and bitumen. Distinguish between hot, containing viscous bitumen, laid and compacted at a temperature not lower than 120 ° C; warm -- with low-viscosity bitumen and compaction temperature of 40-80 °C; cold - with liquid bitumen, compacted at ambient temperature, but not lower than 10 ° C. Asphalt concrete is used for pavements of roads, airfields, sites, etc.
Diesel fuel (solar oil, diesel fuel) is a liquid product used as fuel in a diesel internal combustion engine, as well as in gas diesel engines. Usually, this term is understood as fuel obtained from kerosene-gas oil fractions of direct distillation of oil.
Application: The main consumers of diesel fuel are railway transport, trucks, water transport and agricultural machinery. In addition to diesel and gas-diesel engines, residual diesel fuel (solar oil) is often used as boiler fuel, for impregnating leather, in cutting fluids for mechanical and quenching fluids for heat treatment of metals.
The main characteristics of the fuel: Distinguish low-viscosity distillate - for high-speed, and high-viscosity, residual, for low-speed (tractor, ship, stationary, etc.) engines. The distillate consists of hydrotreated kerosene-gas oil fractions of direct distillation and up to 1/5 of catalytic cracking and coking gas oils. Viscous fuel for low-speed engines is a mixture of fuel oils with kerosene-gas oil fractions. The calorific value of diesel fuel is on average 42624 kJ/kg (10180 kcal/kg).
Physical properties: Summer diesel fuel: Density: not more than 860 kg/m3. Flash point: 62 °C. Pour point: ?5 °C. It is obtained by mixing straight-run, hydrotreated and secondary hydrocarbon fractions with a boiling point of 180--360 degrees Celsius. An increase in the end-of-boil-off temperature leads to increased coking of the injectors and smoke.
Winter diesel fuel: Density: no more than 840 kg/m3. Flash point: 40 °C. Pour point: ?35 °C. It is obtained by mixing straight-run, hydrotreated and recycled hydrocarbon fractions with a boiling point of 180--340 °C. Also, winter diesel fuel is obtained from summer diesel fuel by adding a pour point depressant, which lowers the pour point of the fuel, but slightly changes the limiting filterability temperature. In an artisanal way, up to 20% of kerosene TS-1 or KO is added to summer diesel fuel, while the performance properties practically do not change.
Arctic diesel fuel: Density: no more than 830 kg/m³. Flash point: 35 °C. Pour point: ?50 °C. It is obtained by mixing straight-run, hydrotreated and secondary hydrocarbon fractions with a boiling point of 180--330 degrees Celsius. Boiling limits of arctic fuel roughly correspond to those of kerosene fractions, so this fuel is essentially weighted kerosene. However, pure kerosene has a low cetane number of 35-40 and poor lubricating properties (strong injection pump wear). To eliminate these problems, cetane-boosting additives and mineral motor oil (preferably diesel or KAMAZ) are added to arctic fuel to improve lubricating properties. A more expensive way to produce arctic diesel fuel is dewaxing summer diesel fuel.
Fuel oil (possibly from the Arabic mazkhulat - waste), a dark brown liquid product, the residue after the separation of gasoline, kerosene and gas oil fractions from oil or its secondary processing products, boiling up to 350--360 ° C. Fuel oil is a mixture of hydrocarbons (with a molecular weight of 400 to 1000 g/mol), petroleum resins (with a molecular weight of 500-3000 or more g/mol), asphaltenes, carbenes, carboids and organic compounds containing metals (V, Ni, Fe, Mg, Na, Ca). Physical and chemical properties of fuel oil depend on chemical composition initial oil and the degree of distillation of distillate fractions and are characterized by the following data: viscosity 8--80 mm / s (at 100 ° C), density 0.89--1 g / cm (at 20 ° C), pour point 10--40 °С, sulfur content 0.5--3.5%, ash content up to 0.3%, net calorific value 39.4--40.7 MJ/mol
Fuel oils are used as fuel for steam boilers, boiler plants and industrial furnaces (see Boiler fuels), for the production of marine fuel oil, heavy motor fuel for crosshead diesel engines. The output of fuel oil is about 50% by weight based on the original oil. In connection with the need to deepen its further processing, fuel oil is subjected to further processing on an increasing scale, distilling under vacuum distillates boiling in the range of 350–420, 350–460, 350–500 and 420–500 ° C. Vacuum distillates are used as raw materials for the production of motor fuels, in the processes of catalytic cracking, hydrocracking, and distillate lubricating oils. The residue of vacuum distillation of fuel oil is used for processing at thermal cracking and coking units, in the production of residual lubricating oils and tar, which is then processed into bitumen. The main consumers of fuel oil are industry and housing and communal services.
Gasoline is a mixture of light hydrocarbons with a boiling point of 30 to 200 °C. Density is about 0.75 g/cm. Calorific value approximately 10500 kcal/kg (46 MJ/kg, 34.5 MJ/liter). combustible liquid. Designed for use as a fuel. It is obtained by distillation of oil, hydrocracking and, if necessary, further aromatization - catalytic cracking and reforming. Special gasolines are characterized by additional purification from unwanted components and mixing with useful additives.
Application: At the end of the 19th century, gasoline did not find a better use than an antiseptic (gasoline was sold in pharmacies) and fuel for stoves. Often, only kerosene was distilled from oil, and everything else, including gasoline, was either burned or simply thrown away. However, with the advent of the internal combustion engine operating on the Otto cycle, gasoline has become one of the main products of oil refining. Although, as diesel engines become more widespread, diesel fuel comes to the fore due to the higher efficiency of diesel engines. Gasoline is used as a fuel for carburetor and injection engines, high-pulse rocket fuel (Sintin), in the production of paraffin, as a solvent, as a combustible material, as a raw material for petrochemistry straight-run gasoline or stable gas gasoline (SGS).
Kerosene (English kerosene from Greek kzst - wax) - mixtures of hydrocarbons (from C12 to C15), boiling away in the temperature range of 150-250 ° C, transparent, slightly oily to the touch, combustible liquid obtained by distillation or rectification of oil .
Properties and composition: Density 0.78--0.85 g / cm³ (at 20 ° C), viscosity 1.2 - 4.5 mm2 / s (at 20 ° C), flash point 28-72 ° C, calorific value approx. 43 MJ/kg.
Depending on the chemical composition and method of processing the oil from which kerosene is obtained, its composition includes:
saturated aliphatic hydrocarbons - 20-60%
naphthenic 20-50%
bicyclic aromatic 5-25%
unsaturated - up to 2%
impurities of sulfur, nitrogen or oxygen compounds.
Application: Kerosene is used as a jet fuel, a combustible component of liquid rocket fuel, a fuel for firing glass and porcelain products, for household heating and lighting devices, in metal cutting machines, as a solvent (for example, for applying pesticides), a raw material for the oil refining industry. Kerosene can be used as a substitute for winter and arctic diesel fuel for diesel engines, however, it is necessary to add antiwear and cetane improvers. The cetane number of kerosene is about 40, GOST requires at least 45. For multi-fuel engines (based on diesel), it is possible to use pure kerosene and even AI-80 gasoline . It is allowed to add up to 20% of kerosene to summer diesel fuel to reduce the pour point without degrading performance. It is also used in folk medicine for angina. Also, kerosene is the main fuel for fire shows (fire performances), due to its good absorbency and relatively low combustion temperature. It is also used for washing mechanisms, for removing rust.
Liquefied petroleum gas (LPG) is a mixture of light hydrocarbons compressed under pressure with a boiling point of? 50 to 0 ° C. Designed for use as a fuel. The composition can vary significantly, the main components are propane, propylene, isobutane, isobutylene, n-butane and butylene.
It is produced mainly from associated petroleum gas. It is transported and stored in cylinders and gas holders. It is used for cooking, boiling water, heating, used in lighters, as fuel for vehicles.
Petroleum (mineral) oils are liquid mixtures of high-boiling hydrocarbons (boiling point 300--600 ° C), mainly alkylnaphthenic and alkylaromatic, obtained by oil refining. According to the production method, they are divided into distillate, residual and compounded, obtained, respectively, by distillation of oil, removal of undesirable components from tars, dewaxing, hydrotreating or mixing distillate and residual. Recently, the method of converting the original oil feedstock into more valuable products by hydrocracking has become widespread - the oils obtained in such production, at a much lower cost, are close in properties to synthetic ones.
According to the areas of application, they are divided into lubricating oils, electrical insulating oils, conservation oils. They are also used in the cosmetics industry.
Additives are often added to petroleum oils to impart the desired properties. Based on petroleum oils, plastic and technological lubricants, special fluids, such as cutting fluids, hydraulic fluids, etc., are obtained.
Paraffin is a wax-like substance, a mixture of saturated hydrocarbons (alkanes) of composition from C18H38 to C35H72. The name comes from lat. parum is "little" and athnis is "akin" because of its low receptivity to most reagents. mp 40-65 °С; density 0.880-0.915 g/cm³ (15°C). Obtained mainly from oil.
Properties: Used for the preparation of paraffin paper, impregnation of wood in the match and pencil industries, as part of garden pitch, for finishing fabrics, as an insulating material, chemical raw materials, etc. In medicine, it is used for paraffin treatment. Paraffins are a mixture of solid hydrocarbons of the methane series with a predominantly normal structure with 18-35 carbon atoms per molecule and a melting point of 45-65°C. Paraffins usually contain some isoparaffinic hydrocarbons, as well as hydrocarbons with an aromatic or naphthenic nucleus in the molecule.
Paraffin is a white substance of a crystalline structure with a molecular weight of 300-450, in the molten state it has a low viscosity. The size and shape of paraffin crystals depend on the conditions of its isolation: paraffin is separated from oil in the form of small thin crystals, and from petroleum distillates and distillate raffinates of selective purification - in the form of large crystals. During rapid cooling, the precipitated crystals are smaller than during slow cooling. Paraffins are inert to most chemicals. They are oxidized by nitric acid, atmospheric oxygen (at 140 °C) and some other oxidizing agents to form various fatty acids similar to those found in vegetable and animal fats. Synthetic fatty acid, obtained by the oxidation of paraffin, are used instead of fats of vegetable and animal origin in the perfume industry, in the production of lubricants, detergents and other products.
Paraffins can also be isolated from other products, such as ozokerite. Depending on the fractional composition, melting point and crystal structure, paraffins are divided into liquid (temperature tmelt? 27 ° C), solid (tmelt = 28 - 70 ° C) and microcrystalline (tmelt > 60 - 80 ° C) - ceresins . At the same temperature top. ceresins differ from paraffins in their greater molecular weight, density and viscosity. Ceresins react vigorously with fuming sulfuric acid, with hydrochloric acid, while paraffins react weakly with them. During the distillation of oil, ceresins are concentrated in the sediment, and paraffin is distilled with distillate. Ceresins, which are concentrated in the residue after the distillation of fuel oil, are a mixture of cycloalkanes and in a smaller amount of solid arenes and alkanes. There are relatively few isoalkanes in ceresin. According to the degree of purification, paraffins are divided into gachas (petrolatums), which contain up to 30% (wt.) of oils; crude paraffins (ceresins) with oil content up to 6% (wt.); purified and highly purified paraffins (ceresins). Depending on the depth of cleaning, they are white (highly refined and refined grades) or slightly yellowish and from light yellow to light brown (crude paraffins). Paraffin is characterized by a lamellar or ribbon structure of crystals. Purified paraffin has a density of 881–905 kg/m3. Ceresins are a mixture of hydrocarbons with the number of carbon atoms in the molecule from 36 to 55 (from C36 to C55). They are extracted from natural raw materials (natural ozocerite and residues of highly paraffinic oils obtained during its processing) and synthetically produced from carbon monoxide and hydrogen. Unlike paraffins, ceresins have a finely crystalline structure. Melting point 65--88 °C, molecular weight 500--700. Paraffins are widely used in electrical engineering, food (deep cleaning paraffins; t_melt = 50--54 ° C; oil content 0.5--2.3% by weight), perfumery and other industries. On the basis of ceresin, various compositions are made in the household chemicals industry, vaseline; they are also used as thickeners in the production of greases, insulating materials in electrical and radio engineering and wax mixtures.
Crude solid paraffins are produced by the following methods: 1) deoiling slack and petrolatum - by-products of the production (dewaxing) of oils using solvents (mixtures of ketone, benzene and toluene, dichloroethane), while obtaining crude paraffins (from slack) and ceresins (from petrolatum) ; 2) isolation and deoiling of paraffin from distillates of highly paraffinic oils with a mixture of ketone, benzene and toluene; 3) crystallization of solid paraffins without the use of solvents (by cooling in crystallizers and filter pressing). The crude paraffins are then refined (refined) using acid-base, adsorption (contact or percolation) or hydrogenation refining (to remove unstable substances that stain and smell). Liquid paraffins are isolated from diesel fractions by dewaxing using selective solvents (a mixture of acetone, benzene and toluene), urea dewaxing (in the production of low-solidification diesel fuel) and adsorption on molecular sieves (isolation of liquid C10-C18 paraffins using a porous synthetic zeolite).
Application: candles for lighting, lubricant for rubbing wooden parts (drawer guides, pencil cases, etc.), mixed with gasoline - anti-corrosion coating (flammable!), in cosmetics for the production of vaseline, paraffins are registered as food additives E905x .
Lubricants are solid, plastic, liquid and gaseous substances used in friction units of automotive equipment, industrial machines and mechanisms, as well as in everyday life to reduce wear caused by friction.
The purpose and role of lubricants (lubricants and oils) in technology: Lubricants are widely used in modern technology, in order to reduce friction in moving mechanisms (motors, bearings, gearboxes, etc.), and in order to reduce friction during mechanical processing of structural and other materials on machine tools (turning, milling, grinding, etc.). Depending on the purpose and operating conditions of lubricants (lubricants), they are solid (graphite, molybdenum disulfide, cadmium iodide, tungsten diselenide, hexagonal boron nitride, etc.), semi-solid, semi-liquid (molten metals, greases, constantins, etc. ), liquid (automobile and other machine oils), gaseous (carbon dioxide, nitrogen, inert gases).
Types and types of lubricants: Depending on the characteristics of the materials of the rubbing pair, liquid (for example, mineral, partially synthetic and synthetic oils) and solid (fluoroplastic, graphite, molybdenum disulfide) substances can be used for lubrication.
According to the base material, lubricants are divided into: 1) mineral - they are based on hydrocarbons, oil refining products 2) synthetic - are obtained by synthesis from organic and inorganic (for example, silicone lubricants) raw materials
Classification: All liquid lubricants are divided into viscosity grades (SAE classification for motor and gear oils, ISO VG (viscosity grade) classification for industrial oils), and groups according to the level of performance properties (API, ACEA classifications for motor and gear oils, ISO classification for industrial oils.
By state of aggregation are divided into: 1) solid, 2) semi-solid, 3) semi-liquid, 4) liquid, 5) gaseous.
By appointment: 1) Motor oils - used in internal combustion engines. 2) Transmission and gear oils - used in various gears and gearboxes. 3) Hydraulic oils - used as a working fluid in hydraulic systems. 4) Edible oils and liquids - used in food processing and packaging equipment where there is a risk of contamination of the product by the lubricant. 5) Industrial oils (textile, for rolling mills, hardening, electrical insulating, coolants, and many others) - used in a wide variety of machines and mechanisms for the purpose of lubrication, conservation, sealing, cooling, removal of processing waste, etc. 6) Electrically conductive lubricants ( pastes) - used to protect electrical contacts from corrosion and reduce the contact resistance of contacts. Electrically conductive lubricants are manufactured as greases. 7) Consistent (plastic) lubricants - used in those units in which it is structurally impossible to use liquid lubricants.
3. Basic information about oil
The physical properties of oil vary considerably. Important for the characteristics are: density, viscosity, luminescence, color, smell and others.
The density of oil, like the density of any body, is the mass of oil per unit volume. The density of oil fluctuates on average from 0.75 to 1.00 at a temperature of 20 degrees and depends on the composition of the oil.
Shrinkage coefficient - the value (in percent) of the decrease in the volume of 1 m3 of oil extracted from the reservoir and moved in the conditions of the oil storage. Shrinkage of oil occurs due to the cooling of oil, as well as due to the removal of gas.
Viscosity is the ability of a liquid to resist flow. The higher the viscosity of a liquid, the slower it flows, and vice versa. For example, light oils are very mobile, while heavy oils are very viscous and sometimes turn into semi-solids.
Luminescence is a cold glow of a substance caused by various reasons. The luminescence of a substance under the action of light is called photoluminescence. The last type of luminescence is divided into two subspecies: fluorescence and phosphorescence. Fluorescence is the luminescence of a substance directly when it is irradiated; if, after the cessation of irradiation, the substance continues to glow, then this phenomenon is called phosphorescence.
All oils fluoresce to a greater or lesser extent. Aromatic oils are the most fluorescent. The fluorescence color of gray oils varies from yellow to green and blue. This property is used to determine traces of oil in the rocks traversed by wells, in the so-called luminescent-bitumen survey, during prospecting and exploration.
Optical activity is understood as the ability of organic substances present in oils to rotate the plane of polarization of light. It is due to the presence in the molecule of a substance of an asymmetric carbon atom, that is, an atom, all of whose valences are saturated with various atoms or radicals. The presence of optically active substances in oil is considered, as a rule, one of the proofs of the organic origin of oil, since optically active substances cannot be synthesized organically.
Calorific value is the amount of heat released during the complete combustion of a certain amount of a substance. For example, with complete combustion of 1 kg of oil, 10340-10914 kcal is released, and with complete combustion of 1 m3 of gas - 8900 kcal.
4. Classification and coding of goods
The concept of classification
Classification - the distribution of goods into various groupings based on the combination of goods into these groupings and on the principle of uniformity in the use of the main feature.
Modern world trade uses in its trade turnover, according to experts, 10 to the seventh degree of product names. There are about 200 countries participating in world trade. The World Trade Organization was created to regulate and manage the processes taking place in world trade.
One of the main tasks of the organization is to create a unified global approach, the essence of which is to create a single world language in which all participants in world trade can communicate.
As a single way to create such a language was the possibility of using classifiers. The need for classification of goods arose long ago, it coincided with the appearance of a large number of goods on the markets of Western Europe. At the beginning of attempts to create a classification (18th century), these were primitive lists (lists) of goods, which at that time in some cases bore signs of classification. Food products were classified as overseas and colonial; and non-grocery goods(fabrics, clothes, shoes, jewelry, precious metals and stones, building materials, wood, etc.)
As the economy developed, with the increase in the range of goods on the world market, with the development of factory and factory production, it became necessary to further refine the primary primitive classifications.
Further detailing is based on the use of unifying features, but of lesser significance. The need for detail arose due to the greater need for the nomenclature of goods and led to the creation of modern classifications, first within countries, then to the creation of international classifiers. Modern classifications were created on a scientific basis.
The current state of world trade is unthinkable without managing the trade turnover, assessing its state, creating statistics, studying the market (especially its dynamics), creating customs services, statistical processing of commodity flows, assessing economic characteristics on the scale of world trade. All this is unthinkable without the use of classifications.
Choice of the main feature.
One of the main principles on which the creation of classifications is based are the requirements for the choice of the main feature.
The main sign is the assignment of goods to one or another grouping, which is the basis that unites the nomenclature of goods into one group and which allows, using this sign, to accurately determine the product code in the classification.
When creating classifications, some principles for choosing the main feature are used, while the following are involved:
1. When choosing the main feature, it is recommended to be guided by the origin of the goods. The concept of origin implies uniformity technological processes used in the production of goods in this group. Uniformity should be understood as an industry or type of activity.
2. Means of production are recommended to be classified according to their purpose in the production process. The most characteristic is the division of the classified means of production into means of labor and objects of labor. Objects of labor can be classified using the signs: raw materials, basic materials and auxiliary, as well as fuel (energy sources). When classifying materials on this basis, large groups can be distinguished (building materials, metal products, etc.)
3. Also among the important recommendations can be used the assignment of goods to groupings that unite them on the basis of the uniformity of any properties, and the most important is: the uniformity of physical, chemical and biological properties.
When choosing the main feature that combines goods into a single nomenclature grouping, such characteristics of goods as shapes and sizes, sometimes weight characteristics, can be involved.
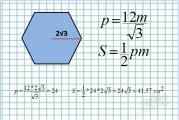
Classification systems.
The practice of creating various classifications most often uses systems based on Arabic or Roman numerical notation systems (most often Arabic). The Arabic numeral system uses decimal and centesimal classification systems. The essence of such systems is that each higher level of classification is divided into either 10 or 100 levels of classification groups. In some cases, these systems can be used simultaneously, but on different levels. This applies only to the use of the Arabic numeral system.
When using Roman digital symbols, these concepts are not acceptable.
In applied, non-scientific, non-legalized classifications, there are no strict requirements for the number of groupings and classification of levels. Such systems do not bear a sign of systematicity (unsystematic). They are called random. A system breakdown occurs when there is not enough capacity in the system at some level of classification.
The disadvantage of the decimal system in some cases is the insufficient capacity of the system when new goods appear, which can lead to the artificial placement of goods in groupings created using an unacceptable main feature - a consequence - the destruction of the system.
The advantages of the decimal system are: compactness, simple digital symbols when coding goods.
The hundredth system is more capacious, avoids the shortcomings of the previous one, but is more cumbersome in construction, has more cumbersome codes (2 digits).
Classification steps.
Within each classification system, goods are distinguished by the number of separate classification levels, called classification levels, depending on how many levels are contained between the concept of “materials” and their specific “size”.
Higher first step - class
Second step - subclass
Third step - group
Fourth step - subgroup
Fifth step - view
The sixth stage is a subspecies (intraspecific classification of the grouping - type and size.
There can be as many intraspecies as required up to a specific weight and size specification of each specific type of goods. With the increase in groupings, the system becomes more complicated. The use of classifiers in practice requires the minimization of these steps (optimization), since with an increase in the number of steps, the number of digital characters in the product code increases.
Optimization consists in finding an agreement between the requirements of compactness and sufficiency, and also the need for reservations for subsequent additions to emerging new products.
The number of groupings depends on the classification nomenclature. When constructing applied classifications (production, warehouse), with small items, 1,2 and 3 step classifications are sufficient.
Classification of petroleum products.
Crude oil is an object of sale, that is, it can be called a commodity, but for the end consumer it is of no interest in its raw form. Therefore, oil is distilled and oil products such as gasolines, ethers, gases, kerosenes, etc. are obtained.
The first barrier to turning crude oil into a marketable product is water. Oil with water forms a stable “water with oil” emulsion, which can only be destroyed by demulsifiers. This is done on ELOU plants. After this process is completed, the distillation of oil begins and the following marketable products are formed:
Table 1
|
Number of carbon atoms in a molecule |
Boiling range |
Application |
|||
|
2. Petroleum ether |
Solvents |
||||
|
Motor fuel, olefin production |
|||||
|
4. Kerosene |
Diesel and jet fuel |
||||
|
5. Gaisol |
|||||
|
6. Lubricating oils |
Lubricants, asphalt. |
Gaseous products are the first distillation fraction. Mainly propane and methane, which are used as fuel.
Petroleum ether - consists of a mixture of pentanes, hexanes and heptanes. It is widely used as a solvent in the food and paint industries.
Gasoline - This gasoline is called straight-run gasoline. It consists predominantly of cyclic and aromatic hydrocarbons. Straight-run gasoline is used as a feedstock for the production of lower hydrocarbons. Gasoline acquires the necessary qualities of fuel when hydrocarbons are introduced into the mixture, appropriate additives and subsequent processing. Kerosene - it is a mixture of saturated and unsaturated hydrocarbons. For many years, kerosene has been used for lighting or has been cracked to make gasoline. Recently, kerosene has been used as fuel for jet engines.
Gas oil and fuel oil - the name itself shows that this fraction was used to enrich water gas when it was used as fuel. Mazut is used in boiler plants operating on liquid fuel.
Lubricating oils - this fraction can be separated by fractionation into oils that differ in viscosity. The viscosity of oils depends on the structure of the hydrocarbons included in the fraction. Lubricants are widely used in various fields of technology to reduce friction of mechanical parts, to protect metal from corrosion. Special additives are added to lubricants to provide them with the desired scope of use.
VAT residue - the residue after the distillation of oil. Composed of hydrocarbons of the asphalt type. Petrolatum, commonly referred to as Vaseline, is obtained from the VAT residue. VAT residue gives asphalt, which is used as a binder in the manufacture of insulating coatings.
coding system.
Encoding is the assignment of an individual cipher or code to a specific product. Any modern level classification system uses a product coding system.
An individual cipher, code, nomenclature number, allow you to avoid misreadings, translations of names from foreign languages.
In the context of the development of world trade, coding allows all participants in this trade, all bodies and services at various levels to uniformly understand and use in the practice of their activities the ciphers and codes of specific goods, or nomenclature groupings.
Uniform digital designations existing all over the world make it possible to achieve this goal. Thus, ciphers and codes in digital form are the only possible language of communication for all participants in world trade.
The use of digital codes allows you to automate all types of work related to specifying product information, and allows you to use computers for this work.
There are a number of requirements for the symbols:
1. Brevity
2. Taking into account the need to digitally transfer complete information about the product
3. Sufficiency, that is, the code is sufficient to specify any product.
4. The need to ensure redundancy, which can ensure the assignment of codes to new products that have appeared on the market.
In practice, the use of codes helps in the creation of classifiers and nomenclatures. The following coding systems can be used: numeric, alphabetic and barcode.
Digital is a method based on assigning a code to a specific type of goods, consisting only of digital designations. The ordinal digital system is used for small nomenclature classifiers of goods. In the order in which the list is created, the product is assigned a sequential number. Serial (more advanced) is used when in large numbers classified goods, its essence lies in the fact that in the classified grouping a series of numbers is distinguished, within the limits there are goods placed according to the essential attribute, according to which the grouping by series is carried out.
The decimal numeric system uses Arabic symbols. Each position, each specific product, each group is allocated a number in the code (from 0 to 9). This figure may indicate a certain classification level, depending on the number of classification steps. This system is the simplest to build and is used very widely. Its advantages: the code is short, simple, clear. Disadvantages: insufficient capacity.
The hundredth digital system consists in assigning a code from 00 to 99 to a specific product. It is used in the hundredth classification system when the number of classes is more than 10, while the capacity is much larger, but the whole system is much more complicated.
Combined system - joint use of decimal and hundredth digital systems at various levels of classification.
The alphanumeric system is used only in applied coding systems, and more often when marking products that are somehow classified. In "serious" classifications, the alphanumeric system is not used.
Classifications do not use bar coding. This is applied coding of goods.
Modern classification systems
Modern classification systems can be built on three principles: hierarchical, faceted and mixed.
The hierarchical principle underlies the construction of the OKP and GS. Its essence is that the classifiers start building from the top of the pyramid. At the top is the largest main feature for the used nomenclature of goods subject to this feature. Further detailing of the features of goods is carried out at lower levels. At different levels, there may be signs that were previously at other levels. A characteristic feature of the hierarchical principle is that at each stage only one attribute can be used, but several times at different levels of the classification model.
All-Russian classifier of products (OKP).
It was created in the USSR. Its creation lasted several decades and continues to this day. The need to create an OKP during the USSR was dictated by the tasks of planned management of the national economy with a number of related tasks, such as the creation of a uniform classifier for the entire industry.
OKP is created on a hierarchical scheme. At the national level, a five-stage system of “Higher Classification Groups” was developed. The task of the VCG is to classify products from the highest levels (industry) to the level of the species, without affecting the intraspecific level (TSR). In turn, industries or ministries instructed the country's enterprises to develop TSR. The final development is not completed and will never be completed.
When creating the OKP at the level of higher groupings, the following grouping was adopted: class, subclass, group, subgroup, species.
Arabic mixed system is used. At the class level, the centesimal is 2 digits, and the subclass, group, subgroup, kind is decimal - 1 digit.
OKP - multi-volume edition. Specific work begins with the industry affiliation of goods, that is, with the definition of the class. Almost always using the OKP, you can define consisting of 6 digits.
When creating the OKP, the following principles were used: at one level, only one attribute can be used, which ensures the uniformity of the interpretation of the classification; possibility of reservation.
Most types of goods, as a rule, can be classified in sufficient detail with 10 digits, but sometimes 5 digits are enough. In this case, the VKG groupings not used in the classification are filled with zeros.
Encoding example.
1. Class 11. Crude oil and gas, services for their extraction, except for survey work (1 1 0 0 0 0 0)
2. Division 1 Crude oil and natural gas (1 1 1 0 0 0 0)
3. Group 1 Crude oil (1 1 1 1 0 0 0)
4. Subgroup 1 Crude crude oil (1111100 - 1111132)
5. Type 1 Oil crude, dehydrated and desalinated (1111210-1111320)
6. Subspecies 1 Crude oil produced, onshore and other (1111131)
harmonized system.
The Harmonized System has a nomenclature, which is essentially a classification. The development of world trade contributed to the creation of the harmonized system and the nomenclature of the harmonized system. At the beginning of the century, a need arose for a unified international classification for solving problems similar to those of the OKP.
Similar Documents
Hygienic properties of goods: definition, indicators, their significance in the evaluation of goods and containers. Acceptance of goods for quality, checking its completeness. Certification of cosmetics, verification and evaluation of its quality, requirements for labeling and packaging, marriage.
control work, added 04/03/2014
Functional properties of food products, their digestibility. Food safety. Inorganic properties and organic properties, food additives used in industry. The essence and significance of food care.
lecture, added 03/21/2010
World market of mineral products. Commodity characteristics of the range of coal, petroleum products and mineral products in the FEACN of the Customs Union. Market conditions for exports and imports. Types of classification of oil and oil products, mineral products and coal.
term paper, added 06/13/2014
State of the cosmetics market in the Russian Federation. Classification and consumer properties of cosmetic products, requirements for their quality. Overview of distribution channels for products and product range. Market segmentation by consumer groups.
term paper, added 01/29/2014
Nutritional, energy, biological and physiological value of food products. Onion vegetables, comparative characteristics of onions and garlic. Classification, range and properties of furniture products. Analysis of standards for metal utensils.
test, added 07/28/2010
Production technology and properties of petroleum products, their classification and scope. Characteristics of LLC "Lukoil-Permnefteprodukt" and its subdivisions. The range of petroleum products sold at gas stations. Ways to improve the activities of the enterprise.
term paper, added 05/11/2014
Goods - one of the most important categories of the market, the result of human interaction with the means of production. The main properties of goods. Use and exchange value of goods. Characteristics of consumer value. Modern classification of goods.
abstract, added 03/01/2011
Property - an objective feature of the product, which manifests itself during its creation, operation or consumption. Physical, chemical and biological properties of goods, methods for their determination. Properties that ensure the safety of products in consumption.
term paper, added 02/13/2012
The ability of a product to meet specific human needs. Indicators characterizing the compliance of the product and its parts with the power and speed capabilities of the body. Properties of pharmaceutical goods and products. Consumer properties of goods.